





Published on Apr 02, 2024
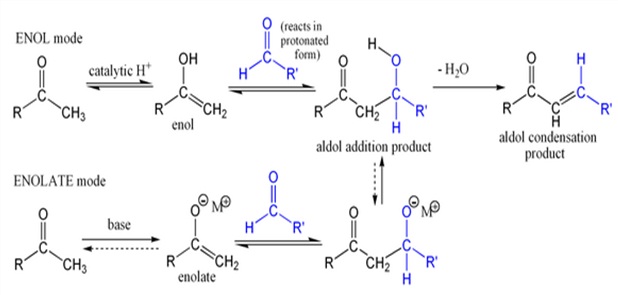
An Aldol Condensation is an organic reaction in which an enolate ion reacts with a carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone, followed by dehydration to give a conjugated enone Aldol condensations are important in organic synthesis, providing a good way to form carbon–carbon bonds. The Robinson annulation reaction sequence features an aldol condensation; the Wieland-Miescher ketone product is an important starting material for many organic syntheses.
Aldol condensations are also commonly discussed in university level organic chemistry classes as a good bond-forming reaction that demonstrates important reaction mechanisms. In its usual form, it involves the nucleophilic addition of a ketone enolate to an aldehyde to form a β-hydroxy ketone, or "aldol" (aldehyde + alcohol), a structural unit found in many naturally occurring molecules and pharmaceuticals.
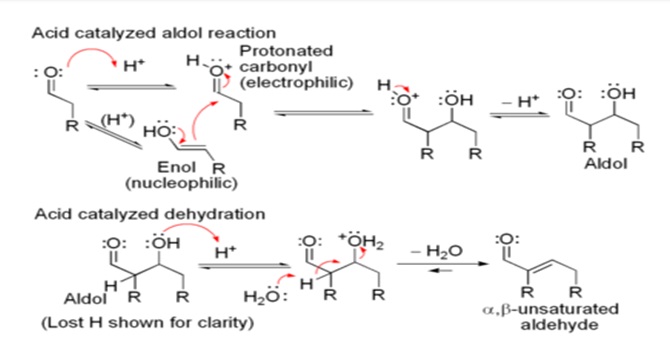
The first part of this reaction is an aldol reaction, the second part a dehydration – an elimination reaction. Dehydration may be accompanied by decarboxylation when an activated carboxyl group is present. The aldol addition product can be dehydrated via two mechanisms; a strong base like potassium t-butoxide, potassium hydroxide or sodium hydride in an enolate mechanism, or in an acid-catalyzed enol mechanism.
In industry the Aldox process developed by Royal Dutch Shell and Exxon, converts propylene and syngas directly to 2-Ethylhexanol via hydroformylation to butyraldehyde, aldol condensation to 2 ethylhexenal and finally hydrogenation.In one study crotonaldehyde is directly converted to 2-ethylhexanal in a palladium / Amberlyst / supercritical carbon dioxide system
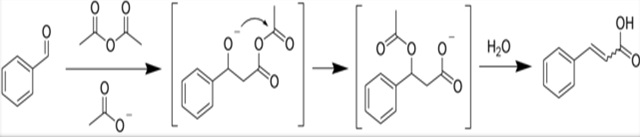
The Perkin reaction is an organic reaction developed by William Henry Perkin that can be used to make cinnamic acids by the aldol condensation of aromatic aldehydes and acid anhydrides in the presence of an alkali salt of the acid
The Meerwein-Ponndorf-Verley Reduction in organic chemistry is the reduction of ketones to secondary alcohols with aluminumisopropylate catalysis in isopropanol solution
Reduction of a ketone 1 to the alcohol 3 in the presence of aluminumisopropylate 2.
First the carbonyl-oxygen coordinates to the aluminumisopropylate 2. This makes the carbonyl-carbon even more positive. In the next step the hydride attacks the carbonyl-carbon and a 6-membered cyclic transition state is formed. After the hydride shift the alcoholate of the desired alcohol 5 and acetone 6 remain. The alcoholate reacts with another isopropanol molecule to form the alcohol 3 and recover the Aluminumisopropylate
All of these reactions are reversible, and complete conversion can be achieved by employing excess alcohol as the reaction solvent or by removal of acetone from the reaction mixture by distillation.
The same process in the opposite direction is called Oppenauer oxidation