





Published on Apr 02, 2024
To Study The Presence Of Oxalate Ion Content In Guava Fruit At Different Stages Of Ripening
Oxalate ions are extracted from the fruit by boiling pulp with dil. H2SO4. Then oxalate ions are estimated volumetrically by the solution with standard KMnO4 solution
100 ml of measuring flask, pestle and mortar, beaker, titration flask, funnel, burette, weight box, filter paper, dilute H2SO4, N\10 KMnO4 solution and guava fruits at different stages of ripening
1. Weigh 50.0 g of fresh guava and crush it to a fine pulp using pestle-mortar.
2. Transfer the crushed pulp to a beaker and add about 50 ml dil. H2SO4 to it. Boil the contents for about 10 minutes.
3. Cool and filter the contents in 100 ml measuring flask. Make the volume up to 100ml by adding distilled water.
4. Take 10 ml of the solution from the measuring flask into a titration flask and add 10ml of H2SO4 to it. Heat the mixture to about 60˚C and titrate it against N/10 KMnO4 solution taken in burette. The end point is appearance of permanent light-pink color.
5. Repeat the above experiment with 50.0 g of 1, 2 and 3 old guava fruit.
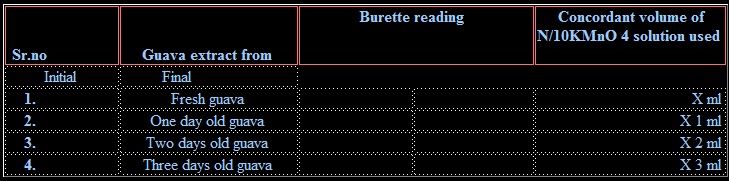
1. for fresh guava
N1V1 = N2V2
(Guava extract) (KMnO4 solution)
N1 ×10 = 1/10 × x = 1/10 ×
Normality of oxalate, N1 = x/100 =
Strength of oxalate in fresh guava extract
= Normality × Eq. mass of oxalate ion
= x/100 × 44g/liter of diluted extract.
= × 44g/liter of diluted extract.
2. for one day old guava
N1V1 = N2V2
(Guava extract) (KMnO4 solution)
N1 ×10 = 1/10 × x = 1/10 ×
Normality of oxalate, N1 = x/100 =
Strength of oxalate in one day old guava extract
= Normality × Eq. mass of oxalate ion
= x/100 × 44g/liter of diluted extract.
= × 44g/liter of diluted extract.
3. for two day old guava
N1V1 = N2V2
(Guava extract) (KMnO4 solution)
N1 ×10 = 1/10 × x = 1/10 ×
Normality of oxalate, N1 = x/100 =
Strength of oxalate in two day old guava extract
= Normality × Eq. mass of oxalate ion
= x/100 × 44g/liter of diluted extract.
= × 44g/liter of diluted extract.
4. for three day old guava
N1V1 = N2V2
(Guava extract) (KMnO4 solution)
N1 ×10 = 1/10 × x = 1/10 ×
Normality of oxalate, N1 = x/100 =
Strength of oxalate in three day old guava extract
= Normality × Eq. mass of oxalate ion
= x/100 × 44g/liter of diluted extract.
= × 44g/liter of diluted extract.
1. PRADEEP’S CHEMISTRY
2. BRITANNICA ENCYCLOPEDIA
3. NCERT CHEMISTRY
4. GOOGLE
5. CHEMISTRY TODAY