





Published on Apr 02, 2024
To Check The Ions Present In The Toothpaste And Determine The Quality.
Every toothpaste contains the following ingredients: binders, abrasives, subsers, humectants, flavours, sweeteners, fluorides, tooth whiteners, a preservative and water. Binders thicken toothpaste- they prevent separation of the solid and liquid component, especially storage. They also effect the speed and volume of foam production, rate of flavor release and product dispersal, the appearance of toothpaste ribbon on the toothbrush. Some binders are gum solid alignate, methyl cellulose, carrageen and magnesium aluminium silicate
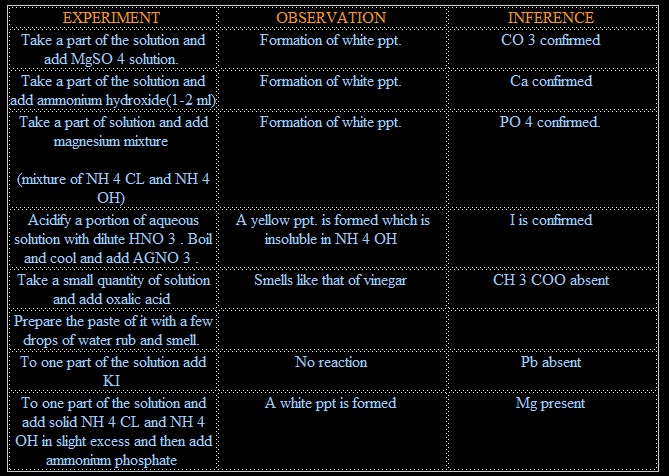
1. CO3 +MgSO4 → MgCO3 + SO4 (white ppt.)
2. CO3 + 2CH3COOH → (CH3COO)2Ca + H2O
(CH3COO)2Ca + (NH4)2C2O4 → 2CHCOONH4 +CaC2O4
3. NaHPO4 + MgCl2+ NH4OH → Mg(NH4)PO4 + 2NaCl + H2O
4. I+ AgNO3 →NO3+AgI (yellow ppt.)
5. (COOH)2 + 2CH3COONa → NO REACTION
6. Pb + 2KI→ NO REACTION
7. MgCl2 + NH4OH+ (NH3)2HPO4 → Mg(NH4)PO4 + 2NH4 +H2O
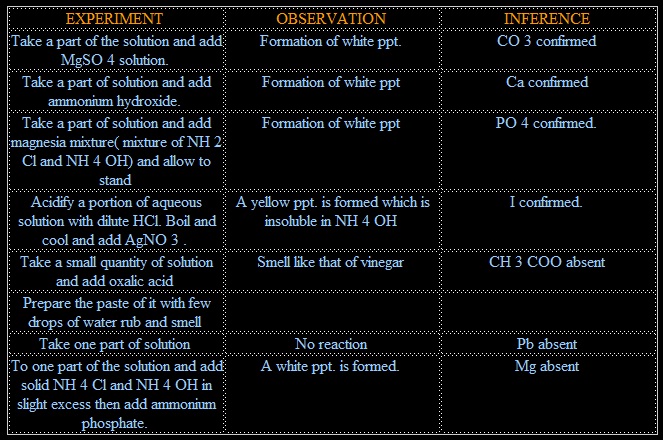
IONS PRESENT:- Mg, I, PO4, Ca, CO3
1. CO3 +MgSO4 → MgCO3 + SO4
(white ppt.)
2. CO3 + 2CH3COOH → (CH3COO)2Ca + H2O
(CH3COO)2Ca + (NH4)2C2O4 → 2CHCOONH4 +CaC2O4
3. NaHPO4 + MgCl2+ NH4OH → Mg(NH4)PO4 + 2NaCl + H2O
4. I+ AgNO3 →NO3+AgI
(yellow ppt.)
5. (COOH)2 + 2CH3COONa → NO REACTION
6. Pb + 2KI→ NO REACTION
7. MgCl2 + NH4OH+ (NH3)2HPO4 → Mg(NH4)PO4 + 2NH4 +H2O
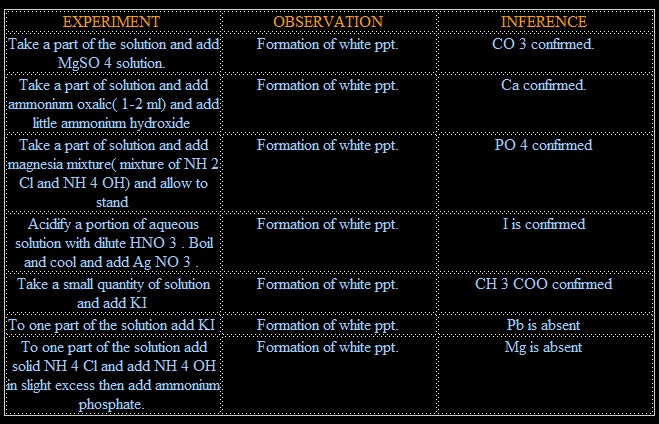
IONS PRESENT:- Mg, I, PO4, Ca, CO3, CH3COO
1. CO3 +MgSO4 → MgCO3 + SO4 (white ppt.)
2. CO3 + 2CH3COOH → (CH3COO)2Ca + H2O
(CH3COO)2Ca + (NH4)2C2O4 → 2CHCOONH4 +CaC2O4
3. NaHPO4 + MgCl2+ NH4OH → Mg(NH4)PO4 + 2NaCl + H2O
4. I+ AgNO3 →NO3+AgI (yellow ppt.)
5. (COOH)2 + 2CH3COONa → NO REACTION
6. Pb + 2KI→ NO REACTION
7. MgCl2 + NH4OH+ (NH3)2HPO4 → Mg(NH4)PO4 + 2NH4 +H2O
Hence after testing different samples of toothpaste, we find that colgate has all necessary for stronger and whiter teeth
1. PRADEEP’S CHEMISTRY
2. BRITANNICA ENCYCLOPEDIA
3. NCERT CHEMISTRY
4. GOOGLE
5. CHEMISTRY TODAY