





Published on Feb 14, 2025
Technology is increasing our energy needs, but it is also showing in new ways to generate power more effetely with less impact on the environment. One of the most promising options for supplementing future power supplies is the fuel cells.
They have the potential to create much more reliable power, with lower levels of undesirable emissions and noise and higher over all efficiency than more traditional power generation systems with existing and projected applications ranging from space craft to private automobiles, large stationary power generator systems to small electronic devices, fuel cells are poised to play an increasingly critical role in meeting the world's plowing demand for clean, reliable power.
Fuel cell is an electrochemical energy conversion device which converts chemicals hydrogen and oxygen to produce electricity by slipping electrons from hydrogen. The hydrogen med is exceeded from natural gun, propane and other common fuel and oxygen is from air.
A fuel cell system consists of 3 major components
1. A fuel cell stack
2. A processor to extract pare hydrogen from the fuel source
3. A storage and conditioners system to adapt the fuel cell's continuous power only out to fluctuating demand.
4. A mechanism for recovering heat from electrochemical process.
The remainder of the system consists of pumps compressors and controls.
Fuel cell stack: in fuel cell stack, purified hydrogen and oxygen from air pass through linked platter similar to those in battery .the electrochemical reaction generator electricity and heat.
An energy storage and power conditioners system adapts the fuel cell's maximum power flour to fluctuating power loads. A battery storage system with dc-ac inventor stores power from low demand periods for use during peak demand .
Heat recovery system directs heat from the jacket of water surrounding the fuel cell in to a preheat tank for the domes tie hot water system.
There are different types of fuel cells
1.Research is underway to develop proton exchange membrane fuel cell.
2.Proton exchange membrane fuel cell user one of the simplest reactions of any fuel cell.
PEM technology was developed after 1960. It was developed for U.S. Navy and Army. The first unit was fueled by hydrogen generated by mixing water and lithium hydride.
The next development in PEM Technology was for NASA's project Gemini in the early days of the U.S. piloted space program .batteries had provided power for earlier missions, but future missions would be longer repairing a different power source.
By mid -1970s PEM cells were developed for under water life support leading to the US nay oxygen generation plant.
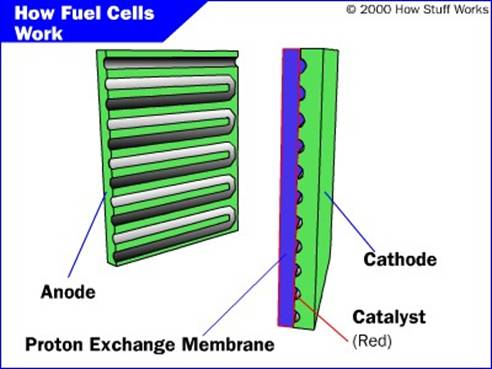
PEM fuel cell has 4 basic components
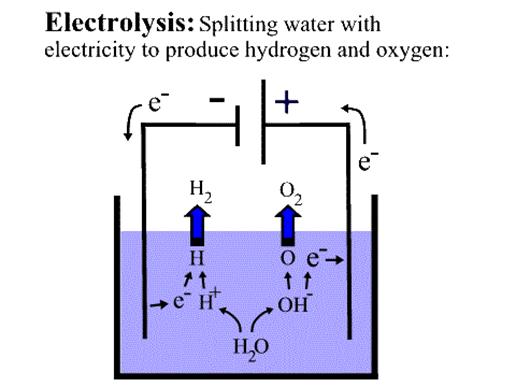
The anode: it is the negative post of the fuel cell has several jobs .it conducts electrons that are freed from the hydrogen molecular so that they can be used in an external circuit. It was channels etched into if that disperse the hydrogen gun equally over the surface of the catalyst
The cathode: it is the positive post of the fuel cell has channels etched in to if that distribute the oxygen to the surface of the catalyst it also conducts the
Electrons back from the external circuit to the catalyst, where they can recombine with the hydrogen cons and oxygen to form water.
The electrolyte: electrolyte is proton exchange membrane. The membrane is made from “nafion”, a sulfate polymer made by Dupont
The catalyst: the catalyst is a special material that facilitates the reaction of oxygen and hydrogen. The usually used catalyst is platinum powder very thirty coaled on to carbon paper or cloth. The carbon is electrically neutral but conductive and also porous allowing the flow of gun and cons through it platinum coated side of the catalyst faces the PEM
The pressurized hydrogen gas is entering the fuel on the anode side. This gun is forced through the catalyst by pressure. When Hz molecule comes in contact with the platinum on the catalyst, it splits on to two H+ cons and two electrons. The proton then travels through th membrane to the side of the fuel cell. But the electron can not permanently through the membrane. Instead it travels through an electric wire to get to the other side, and delivers its energy to a load along the way, such as a bulb .mean while on the cathode side of the fuel cell, oxygen gas (O2) is being forced through the catalyst where it forms oxygen atoms. Each of these atoms has a strong –ve change.
The –ve charge attracts two H0 cons through the membrane where they recombine to oxygen atom and two water molecules are formed.
The reaction taking place is
Anode side:
2H2> 4H+ + 4e-
Cathode side:
O2 + 4H+ + 4e- >2H2O
Net reaction:
2H2 + O2 > 2H2O + energy (electricity)
This reaction in a single fuel cell produces only about +ve but the voltage provided by each fuel cell that is large enough for practical application. Many fuel cells can be combined to form a fuel cell stack. The figure below shows a fuel cell stack
PEMIC operate at family low temperature (about 1760 F or 800 C), which means they watch up quickly and don’t require expensive containment structures. It has high power density and can vary their output quickly to meet shifts on power demand.
PEM fuel cells are relatively smaller size, low material cost, high performance and high volume manufacturability make them ideal for transportation, stationary and portable applications.
MCFCs are so named because the electrolyte they use is a molten alkali carbonate mixture, retained in a matrix. They operate at a temperature of about 650°C, meaning that once again useful heat is produced. In this case the cathode must be supplied with carbon dioxide, which reacts with the oxygen and electrons to form carbonate ions, which carry the ionic current through the electrolyte. At the anode these ions are consumed in the oxidation of hydrogen, which also forms water vapour and carbon dioxide to be transferred back to the cathode. There are two ways of doing this: either by burning the anode exhaust with excess air and removing the water vapour before mixing it with the cathode inlet gas; or by separating the CO2 from the exhaust gas using a 'product exchange device'.
The fuel consumed in an MCFC is usually natural gas, though this must be reformed in some way to create a hydrogen-rich gas to feed to the stack. An MCFC produces heat and water vapour at the anode, which can be used for the steam reformation of methane. This means that it is inherently more efficient than a cell requiring external fuel processing. Again, the MCFC can use carbon monoxide at the anode as a fuel.
The MCFC is seen by many as an ideal source for large scale power generation. One reason for this is the necessity for large amounts of ancillary equipment, which would render a small operation uneconomic. There is also no requirement for expensive catalysts as in low temperature fuel cells, and a third reason is the fact that the heat generated can be used for internal reformation of methane, a bottoming cycle and for fuel processing and cogeneration. This increases the efficiency of the fuel cell system.
| Are you interested in this topic.Then mail to us immediately to get the full report.
email :- contactv2@gmail.com |