





Published on Apr 02, 2024
When a glucose solution is poured into the white cubes, the Walkman begins to play. When an isotonic drink is poured in, a propeller starts to spin. In the summer of 2007, the Sony-developed bio battery was announced in newspapers, magazines, and TV reports, and evoked a strong response. Carbohydrates (glucose) are broken down to release energy and generate electricity.
This bio battery, which is based on mechanisms used in living organism, is not only friendly to the environment but also has great potential for use as an energy source.
This prototype bio battery has achieved the world’s highest power output of 50 mW*2 when employed for a passive type*1 system. These research results were published at the 234th American Chemical Society National Meeting & Exposition in August 2007 and earned respect from an academic point of view.
Sony successfully demonstrated bio battery powered music playback with a memory type Walkman and passive speakers (which operate on power supplied by the Walkman) by connecting four bio battery units in series. The case of this bio battery, which is made from an organic plastic (polylactate), is designed to be reminiscent of a living cell.
Plants create both carbohydrates and oxygen by photosynthesis from carbon dioxide and water. Animals take up those carbohydrates and oxygen and utilize them as an energy source and release carbon dioxide and water. Then this cycle starts again. Since the carbon dioxide is recycled in this system, the amount of carbon dioxide in the atmosphere does not increase. If electrical energy could be directly acquired from this cycle, we could obtain more environmentally friendly energy than that from fossil fuels. Furthermore, renewable energy sources such as glucose (which is present in plants and therefore abundantly available) have an extremely high energy density. One bowl of rice (about 100 grams) is equivalent to 160 kilocalories, which corresponds to the energy about 64 AA alkaline dry cells.
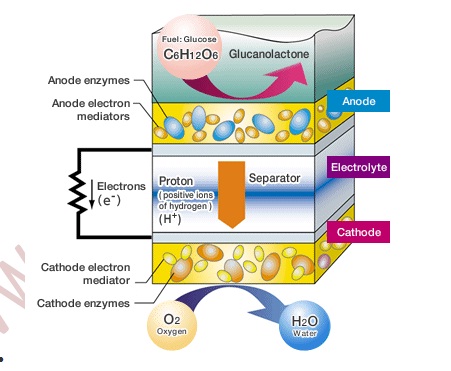
Therefore, this bio battery, which is based on Energy for activity, that is the ATP and thermal energy commonly used in the living organism, can be obtained from the exchange of the electrons and protons through these two enzymatic reactions. To take advantage of this living organism mechanism, the energy for activity from inside the organism must be removed outside the organism as electrical energy.
That is, when the electrons and protons move from enzyme to enzyme, it is necessary to extract just the electrons and divert them through a separate path. Thus Sony used an electron transport mediator so that electrons could be exchanged smoothly between the enzymes and the electrodes that are the entrance and exit to that detour. The principles of the bio battery are based on the energy conversion mechanism in living organisms. However, in order to create the bio battery, several technologies needed to be developed.
These include immobilization of enzymes that are normally incompatible with carbon and metal electrodes, electrode structures, and electrolytes. mechanisms used in living organisms, is not only friendly to the environment but is also likely to be of practical use as an energy source. Sony has focused on these advantages since 2001 and has developed an electrical power generation device that uses mechanisms similar to those in living organisms.
Like a conventional fuel cell battery, Bio Battery basically consists of an anode, cathode, electrolyte and separator. However, Bio Battery has certain specific characteristics. First, biological enzymes are used as catalysts for the anode and cathode. Second, enzymes and electronic mediators (which transfer electrons between enzymes, and between enzymes and electrodes) are fixed on the anode and cathode.
Several potential applications of BFCs have been reported or proposed in the literature for implantable devices, remote sensing and communication devices as a sustainable and renewable power source. However, there are no BFC design formats or templates that allow for the production of a working device with a size on the order of 1 cc, which are needed for several “real world” applications. An enzyme based BFC is very attractive, however it has been shown that electron flow is too slow to make a viable fuel cell. This is due to the difficulty for enzymes to attain direct electrical contact with the electrodes of the cell and catalyze reactions effectively. The two largest obstacles with bio-fuel cells which must be overcome are increasing the power density and increasing the enzyme stability.
In addition, understanding of the determinants governing the direct electron transfer reac- tion and mutation of enzymes to tune the redox potential, to improve DET kinetics, or to reduce the enzyme size are also very important challenges facing the commercialization of bio-fuel cells.15 To address these key issues, various enzyme immobilization methods have been attempted for constructing BFCs, such as adsorption, entrapment, and covalent attachment.
Recent advances in bionanotechnology are promising to improve the performance and stability of immobilized enzymes beyond the scope of these traditional approaches.16 The large surface area pro- vided by nanomaterials for the attachment of enzymes will increase enzyme loading and possibly improve the power density of BFCs. Additionally, various nanostruc- tured materials have shown great potential for stabilizing enzyme activity, which can be further employed in improv- ing the lifetime of BFCs.
Bio-fuel cells are attracting increased attention mainly due to promising advances from the research laboratories around the world resolved before bio-fuel cells become commercially viable for practical applications. The main challenges are: (1) Nanostructured bioelectrocatalysis. (2) Immobilization of bioelectrocatalysts. (3) Protein denaturation induced by CNT. The following sections briefly describe these issues:
Traditional direct hydrogen fuel cells require noble metal catalysts both for hydrogen oxidation and oxygen reduction.17 Similarly, the bio-fuel cells also need catalysts (bio-catalysts) for the conversion of chemical to electrical energy. One approach is to use microorganisms and/or enzymes as biological reactors for the fermentation of raw materials to fuel products (similar hydrogen fuel reform- ers); the second approach is to use the microorganisms and/or enzymes as catalysts directly in the bio-fuel cells.

The second approach, using purified redox enzymes for the targeted oxidation and reduction of specific fuel and oxidizer substrates, is more efficient for bio-fuel cells. Also, bio-catalysts are an attractive renewable and less expensive alternative to transition metal catalysts for mediated electron transfer (MET).18 MET-type bioelectrocatalyt based BFCs offer the cur- rent density advantage over the direct electron transfer (DET) type, but require that mediators and enzymes be immobilized on electrode surfaces. The construction of DET-type bio-fuel cell is relatively simple as the sys- tem is free from several restrictions concerning mediators.
The cell would not require separators because the crossover of fuels (substrates) would not occur in principle due to enzymatic substrate specificity as long as the enzymes are immobilized on electrodes and dehydrogenases (that is, redox enzymes reacting with electron acceptors except dioxygen) are utilized as anode catalysts. Kamitaka’s group have reported a construction of sin- gle compartment bio-fuel cell, with no separators, using D-fructose dehydrogenase (FDH) from Gluconobacter sp. and laccase from Trametes sp. (TsLAC) as DET-type bio- electrocatalysts in the two-electron oxidation of D-fructose and four-electron reduction of oxygen in the anode and cathode, respectively.
There is also a recent study of utilizing tungsten carbide as an electrocatalyst towards the oxidation of several common microbial fermentation products (hydrogen, for- mate, lactate and ethanol) for microbial fuel cell conditions (e.g., pH 5 at ambient temperature and pressure). Current densities of up to 8.8 mA cm−2 are achieved for hydrogen (hydrogen saturated electrolyte solution), and up to 2 mA cm−2 for formate and lactate, respectively, with cell voltage values between 0.15 and 0.3 V.20 It is also worth mentioning virus-based lithium-ion bio- batteries. There is an increasing need for smaller and more flexible Li ion batteries and for methods to assemble battery materials in various applications. However, realizing smaller and flexible battery systems need monodisperse, homogeneous nanomaterials and hierarchical organization control.
| Are you interested in this topic.Then mail to us immediately to get the full report.
email :- contactv2@gmail.com |