





Published on Apr 02, 2024
Aim is to study the Discoveries In The Field Of Chemistry
Oxygen had been produced by several chemists prior to its discovery in 1774, but they failed to recognize it as a distinct element. Joseph Priestley and Carl Wilhelm Scheele both independently discovered oxygen, but Priestly is usually given credit for the discovery. They were both able to produce oxygen by heating mercuric oxide (HgO). Priestley called the gas produced in his experiments 'dephlogisticated air' and Scheele called his 'fire air'. The name oxygen was created by Antoine Lavoisier who incorrectly believed that oxygen was necessary to form all acids.
Oxygen is the third most abundant element in the universe and makes up nearly 21% of the earth's atmosphere. Oxygen accounts for nearly half of the mass of the earth's crust, two thirds of the mass of the human body and nine tenths of the mass of water. Large amounts of oxygen can be extracted from liquefied air through a process known as fractional distillation. Oxygen can also be produced through the electrolysis of water or by heating potassium chlorate (KClO3).
Oxygen is a highly reactive element and is capable of combining with most other elements. It is required by most living organisms and for most forms of combustion. Impurities in molten pig iron are burned away with streams of high pressure oxygen to produce steel. Oxygen can also be combined with acetylene (C2H2) to produce an extremely hot flame used for welding. Liquid oxygen, when combined with liquid hydrogen, makes an excellent rocket fuel. Ozone (O3) forms a thin, protective layer around the earth that shields the surface from the sun's ultraviolet radiation. Oxygen is also a component of hundreds of thousands of organic compounds.
Atomic Number: 8
Atomic Weight: 15.9994
• 1803 Dalton - the atom is a indivisible, indestructible, tiny ball
• 1850 Evidence is accumulating that the atom is itself composed of smaller particles
• The current model...
The behavior of electrically charged particles Like charges repel each other, unlike charges attract
• A charged particle moving though a magnetic field will feel a force perpendicular to the plane described by the velocity vector and magnetic field vector
• This deflects the moving charged particle according to the "right hand rule" (based on a positive charge)
• A negative charge will be deflected in the opposite direction
Electrical discharge through partially evacuated tubes produced radiation. This radiation originated from the negative electrode, known as the cathode (thus, these rays were termed cathode rays).
• The "rays" traveled towards, or were attracted to the positive electrode (anode)
• Not directly visible but could be detected by their ability to cause other materials to glow, or fluoresce
• Traveled in a straight line
• Their path could be "bent" by the influence of magnetic or electrical fields
• A metal plate in the path of the "cathode rays" acquired a negative charge
• The "cathode rays" produced by cathodes of different materials appeared to have the same properties
These observations indicated that the cathode ray radiation was composed of negatively charged particles (now known as electrons).J.J. Thompson (1897) measured the charge to mass ratio for a stream of electrons (using a cathode ray tube apparatus) at 1.76 x 108 coulombs/gram.
• Charged particle stream can be deflected by both an electric charge and by a magnetic field
• An electric field can be used to compensate for the magnetic deflection - the resulting beam thus behaves as if it were neutral
• The required current needed to "neutralize" the magnetic field indicates the charge of the beam
• The loss of mass of the cathode indicated the "mass" of the stream of electrons
Urea (also known as carbamide) is a waste product of many living organisms, and is the major organic component of human urine. This is because it is at the end of chain of reactions which break down the amino acids that make up proteins. These amino acids are metabolized and converted in the liver to ammonia, CO2, water and energy. But the ammonia is toxic to cells, and so must be excreted from the body. Aquatic creatures, such as fish, can expel the ammonia directly into the water, but land-based animals need another disposal method. So the liver converts the ammonia to a non-toxic compound, urea, which can then be safely transported in the blood to the kidneys, where it is eliminated in urine.
Urea has quite an interesting history. It was first discovered and isolated from human urine by H.M. Rouelle in 1773, and was then successfully synthesized in 1828 by Friedrich Wohler. The synthesis was almost an accident, as Wohler had been trying to make another compound, ammonium cyanate, to continue a study of cyanates he had been working on for the previous few years. When he added silver cyanate to ammonium chloride solution he obtained a white crystalline material, which proved identical to urea obtained from urine.
This discovery was very important, as this made urea the first organic compound to be synthesized from wholly inorganic starting materials. Wohler wrote triumphantly to Berzelius:
"I must tell you that I can make urea without the use of kidneys, either man or dog. Ammonium cyanate is urea."
This discovery dealt a severe blow to a widespread belief at the time called "vitalism". This theory maintained that living organisms, like plants and animals, were made of different materials to inanimate objects like rocks. The belief was that living organisms possessed an unknown 'vital force' that allowed them to fabricate organic chemicals, and since inanimate objects did not possess this force, they could neither create, nor be transformed into the chemicals of life. Wohler's discovery showed that not only could organic chemicals be modified by chemistry, but that they could also be produced through chemistry as well. In effect, he had shown that we are made of the same materials as the rest of Nature, and are therefore a part of the world around us
Humphrey Davy was born on December 17, 1778 in Penzance, Cornwall, England. He received his education in Penzance and in Truro. His father died in 1794, and Davy, in an effort to help support his family, became an apprentice to a surgeon-apothecary. Davy's most important investigations were devoted to electrochemistry. Following Galvani's experiments and the discovery of the voltaic pile, interest in galvanic electricity had become widespread. The first chemical decomposition by means of the pile was carried out in 1800 by Nicholson and Carlisle, who obtained hydrogen and oxygen from water, and who decomposed the aqueous solutions of a variety of common salts. Davy, too, began to example the chemical effects of electricity in 1800
He soon found that when he passed electrical current through some substances, these substances decomposed, (a process later called electrolysis). In 1813, Sir Humphrey Davy concocted a giant battery in the basement of Britain's Royal Society. It was made of 2,000 pairs of plates and took up 889 square feet. The intensity of its effect (the voltage generated) was directly related to the reactivity of the electrolyte with the metal. Evidently, Davy understood that the actions of electrolysis and of the voltaic pile were the same. His work led him to propose that the elements of a chemical compound are held together by electrical forces. Davy must have known of Lavoisier's suggestion that the alkali earths were s of unknown metals. At first, he tried to separate the metals by electrolyzing aqueous solutions of the alkalis, but this yielded only hydrogen gas.
He then tried passing current through molten compounds, and his persistence was rewarded when he was able to separate globules of pure metal by this means. His first successes came in 1807 with the separation of potassium from molten potash and of sodium from common salt. He described potassium as particles which, when thrown into water, "skimmed about excitedly with a hissing sound, and soon burned with a lovely lavender light." Dr. John Davy, Humphrey’s brother, said that Humphreys "danced around and was delirious with joy" at his discovery. These results were presented in the Bakerian lecture of November, 180
The one who put the stop to the dispute was an English scientist named Joseph John Thomson. In his experiment to produce cathode rays he used a tube filled with rarefied gas but he made a bit of modification with the equipment. As it was said before cathode rays are emitted from the cathode and directed to the anode. What Thomson made with the equipment was a little gap in the anode. Through the gap a small beam of cathode rays got out of the area of the cathode and anode influence. Next, the beam passed through a long vacuum tube and fell on a fluoroscopic screen leaving there a fluorescent sign. In the vacuum tube Thomson put also two metal plates connected to a battery. That way he could create voltage between the plates, where the beam had its path. The field was directed perpendicularly to the cathode rays beam. It emerged that under the influence of voltage the beam was deflected (the spot on the screen appeared in a different place than without the voltage turned on). It was the final evidence that cathode rays consisted of charged particles- the other way the beam couldn't be deflected by the electric field. The direction of the deflection has shown of what charge the particles creating the beam are. It emerged to be the negative chargeare. It emerged to be the negative charge.
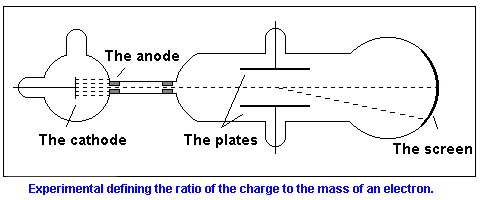
Knowing that cathode rays were formed of charged particles Thomson decided to measure the velocity of those particles. Except from the electric field he used the magnetic one. The deflection of a particle in the magnetic field depends on the velocity of the particle. Arranging the electric and the magnetic field leveling each other in their influence on the particles, and knowing the intensities of both fields one can calculate the velocity of the particles of cathode rays. That is what Thomson did
In 1896 Henri Becquerel was using naturally fluorescent minerals to study the properties of x-rays, which had been discovered in 1895 by Wilhelm Roentgen. He exposed potassium uranyl sulfate to sunlight and then placed it on photographic plates wrapped in black paper, believing that the uranium absorbed the sun’s energy and then emitted it as x-rays. This hypothesis was disproved on the 26th-27th of February, when his experiment "failed" because it was overcast in Paris. For some reason, Becquerel decided to develop his photographic plates anyway. To his surprise, the images were strong and clear, proving that the uranium emitted radiation without an external source of energy such as the sun. Becquerel had discovered radioactivity.
Becquerel used an apparatus similar to that displayed below to show that the radiation he discovered could not be x-rays. X-rays are neutral and cannot be bent in a magnetic field. The new radiation was bent by the magnetic field so that the radiation must be charged and different than x-rays. When different radioactive substances were put in the magnetic field, they deflected in different directions or not at all, showing that there were three classes of radioactivity: negative, positive, and electrically neutral
In the United States during the 1860s, John Wesley Hyatt experimented with cellulose nitrate. In 1865, Hyatt became involved in devising a method for producing billiard balls from materials other than ivory. Originally using mixtures of cloth, ivory dust, and shellac, he patented in 1869 the use of collodion for coating billiard balls. The patent came one year after his collodion material was introduced commercially.
John W. Hyatt and his brother Isaiah took out U.S. Patent 105,338 in 1870 for a process of producing a horn-like material using cellulose nitrate and camphor. Although Parkes and Spill had mentioned camphor in their work, the Hyatt brothers recognized the value of camphor as a plasticizer for cellulose nitrate. In 1872, the term “celluloid” was coined by Isaiah Hyatt to describe the Hyatt’s’ commercially successful product.
The validity of Hyatt’s’ patents was challenged by Spill, and a number of court actions took place between 1877 and 1884. In the final action, it was found that Spill had no claim on the Hyatt brothers’ patents, the judge ruling that Parkes was the true inventor of the process because he had mentioned the use of camphor in his patents. Thus, there was no restriction on the use of these processes and any company, including the Hyatt’s’ Celluloid Manufacturing Company, was free to use them. After that decision, the Celluloid Manufacturing Company prospered, changed its name to the American Cellulose Chemical Corporation, and eventually was absorbed by the Celanese Corporation