





Published on Apr 02, 2024
Aim is to find the Content of Cold Drinks Available in the Market.
Cold drinks of different brands are composed of alcohol, carbohydrates, carbon dioxide phosphate ions etc. These softdrinks give feeling of warmth, lightness and have a tangy tastewhich is liked by everyone. Carbon dioxide is responsible for theformation of froth on shaking the bottle.
The carbon dioxide gas is dissolved in water to form carbonic acidwhich is also responsible for the tangy taste. Carbohydrates are thenaturally occurring organic compounds and are major source ofenergy to our body. General formula of carbohydrates isCX (H2O)Y.
On the basis of their molecule size carbohydrates are classified as:- Monosaccharide, Disaccharides and Polysaccharides. Glucose is a monosaccharide with formula C6H12O6 .It occurs in Free State inthe ripen grapes in bones and also in many sweet fruits. It is alsopresent in human blood to the extent of about 0.1%. Sucrose is oneof the most useful disaccharides in our daily life.
It is widelydistributed in nature in juices, seeds and also in flowers of manyplants. The main source of sucrose is sugar cane juice whichcontain 15-20 % sucrose and sugar beet which has about 10-17 %sucrose. The molecular formula of sucrose is C12H22O11. It isproduced by a mixture of glucose and free dose. It is non-reducingin nature whereas glucose is reducing. Cold drinks are a bit acidicin nature and their acidity can be measured by finding their pHvalue. The pH values also depend upon the acidic contents such ascitric acid and phosphoric acid
1-2 drops of the sample of cold drink of each brand was taken and put on the pH paper. The change in the color of pH paper was noticed and was compared with the standard pH scale
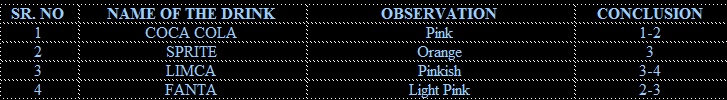
Soft drinks are generally acidic because of the presence of citric acid and phosphoric acid. pH values of cold drink of different brands are different due to the variation in amount of acidic contents
As soon as the bottles were opened, one by one the sample was passed through lime water. The lime water turned milky.
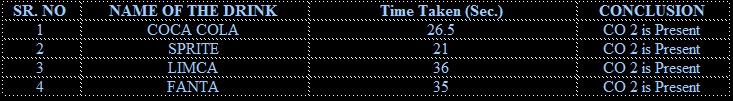
All the soft drinks contain dissolved carbon dioxide in water. The carbon dioxide (CO2) dissolves in water to form carbonic acid, which is responsible for its tangy taste.
CHEMICAL REACTION INVOLVED
Ca(OH)2 (s) + CO2(g) ---> CaCO3 (s) + H2O(s)
Glucose is a reducing sugar acid. Its presence is detected by the following test:-
A small sample of cold drink of different brands was taken in a test tube anda few drops of Benedict’s reagent were added. The test tube was heated for few seconds. Formation of reddish color confirms the presence of glucose in cold drinks

INFERENCE All the samples gave positive test for glucose with Benedict’s reagent. Hence all the drinks contain glucose
Samples of each brand of cold drinks are taken in sample test tube and iodine followed by potassium iodide and sodium hydroxide (NaOH) solution is added to each test tube. Then the test tube are heated in hot water bath for 30 minutes yellow colored precipitate confirmed the presence of alcohol in cold drinks
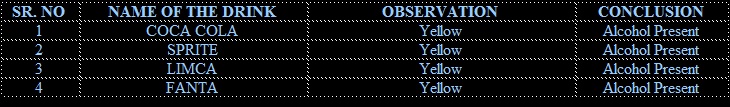
• After conducting several tests, it was concluded that the different brands of cold drinks namely 1. Coca cola 2. Sprite 3. Limca 4. Fanta All contains glucose, alcohol sucrose, phosphate, ions and carbon dioxide. All are acidic in nature.
On comparing the pH value of different brands coca cola is most acidic and limca is least acidic of all the four brands taken. pH value of coca cola is nearly equal to disinfectant which is harmful for body.
CARBON DIOXIDE Among the four samples of cold drinks taken –sprite has maximum amount of dissolved carbon dioxide and Fanta hasminimum amount of dissolved carbon dioxide.
1. Soft drinks are little more harmful than sugar solution. As theycontain sugar in large amount which cause “diabetes”.
2. Soft drinks can cause weight gain as they interfere with the body’s natural ability to suppress hunger feeling.
3. Soft drinks have ability to dissolve the calcium so they are also harmful for our bones.
4. Soft drinks contain “phosphoric acid” which has a pH of 2.8. So they can dissolve a nail in about 4 days.
5. For transportation of soft drinks syrup the commercial truck must use the hazardous matter place cards reserved for highly consive material.
6. Soft drinks have also ability to remove blood so they are very harmful to our body.
1. Cold drinks can be used as toilet cleaners.
2. They can remove rust spots from chrome car humpers.
3. They clean corrosion from car battery terminals.
4. Soft drinks are used as an excellent ‘detergent’ to remove grease from clothes.
5. They can lose a rusted bolt.