https //phet.colorado.edu/sims/html/build-an-atom/latest/build-an-atom en.html
The Build an Atom interactive simulation from PhET allows you to explore the structure of atoms and understand how their properties are determined by the arrangement of protons, neutrons, and electrons.
Here’s how to access and use the simulation:
1. Go to the PhET website: Open a web browser and navigate to the PhET Interactive Simulations website: https://phet.colorado.edu/en/simulations/build-an-atom
2. Click on the “Build an Atom” simulation: The simulation will open in a new window.
3. Explore the interface:
- The simulation window displays a periodic table of elements on the left side.
- On the right side, you’ll find a central area where you can build atoms by dragging and dropping protons, neutrons, and electrons into the nucleus.
- Below the nucleus, there are sliders to adjust the number of protons, neutrons, and electrons.
- At the bottom, you’ll see information about the element you’ve built, including its name, symbol, atomic number, mass number, and charge.
4. Build an atom:
- Start by selecting an element from the periodic table. Click on its symbol to highlight it.
- Then, drag protons, neutrons, and electrons from the right side into the nucleus. Remember that the number of protons determines the element, while the combination of protons and neutrons determines the mass number, and the balance of protons and electrons determines the charge.
- As you build the atom, the information at the bottom will update to reflect the changes.
5. Experiment and observe:
- Change the number of protons, neutrons, and electrons to see how the element and its properties change.
- Observe how the atomic number, mass number, and charge are affected by these changes.
- Explore the different isotopes of an element by changing the number of neutrons while keeping the number of protons constant.
- Create ions by adding or removing electrons to give the atom a positive or negative charge.
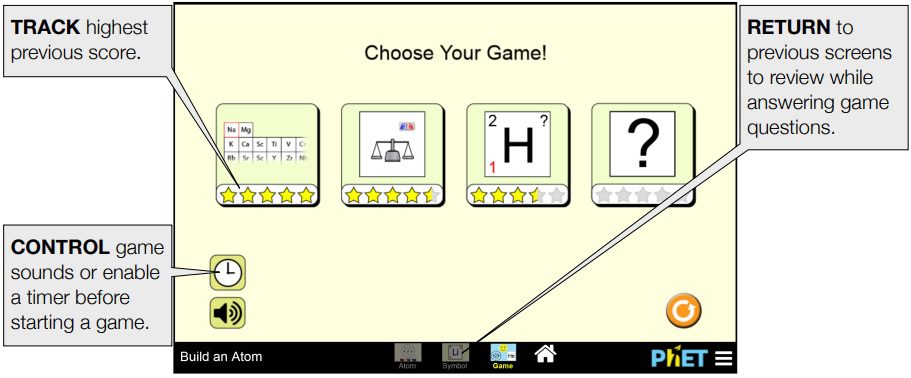
6. Play the game:
- The simulation includes a fun game where you can test your understanding of atomic structure. Click on the “Game” tab at the top of the window to access it.
Sample Learning Goals:
- Use the number of protons, neutrons, and electrons to draw a model of the atom, identify the element, and determine the mass and charge.
- Predict how addition or subtraction of a proton, neutron, or electron will change the element, the charge, and the mass.
- Use the element name, mass, and charge to determine the number of protons, neutrons, and electrons.
- Define proton, neutron, electron, atom, and ion.
- Generate an isotopic symbol for an atom, given the number of protons, neutrons, and electrons
aainflight.com  Login to Watch Movies: What’s On My AA Flight?
Model Simplifications
• Although the title of the sim is “Build an Atom”, students can build both neutral atoms and ions.
• The nucleus is magnified to allow students to see the number of protons and neutrons.
• The radii of the orbits in the Bohr model are not in the correct ratio.
• In the “Cloud” model, the shape of the cloud is not meant to represent orbitals and the size of the cloud does not represent actual atomic or ionic radii. The cloud simply gets larger and darker as the number of electrons in the cloud increases.
• We define “Stable” as an isotope whose half-life is too long to be measured. The nucleus of an “Unstable” atom vibrates but does not fall apart.
• Students can create ions that are not found in nature (for example, He+2). Students can still reach suggested learning goals related to net charge on ions even if not all ions they create exist in nature.
• Excited states are not allowed in the sim. In the Bohr model, if a core electron is removed, an outer electron will move to the inner shell. The sim does not show the subsequent release of a photon due to this electron movement.
• The Symbol representation uses the standard isotope notation with the mass number on the top and the atomic number on the bottom. This may differ from how the data is displayed in some periodic tables (atomic number at the top). Students who are unfamiliar with the isotope notation may require additional scaffolding.
Bankmobile Vibe Activate Card : How do I Activate my BankMobile card?
Game Screen
Students are presented with 5 challenge questions in each game.
Game 1 – Identify the element when provided a model or count of subatomic particles.
Game 2 – Calculate the mass number or charge of an atom or ion.
Game 3 – Interpret atomic symbols
Game 4 – Mixed review
Be the first to comment