





Published on Apr 02, 2024
A paper battery is a flexible, ultra-thin energy storage and production device formed by combining carbon nanotube s with a conventional sheet of cellulose-based paper. A paper battery acts as both a high-energy battery and supercapacitor , combining two components that are separate in traditional electronics .
This combination allows the battery to provide both long-term, steady power production and bursts of energy. Non-toxic, flexible paper batteries have the potential to power the next generation of electronics, medical devices and hybrid vehicles, allowing for radical new designs and medical technologies.
Paper batteries may be folded, cut or otherwise shaped for different applications without any loss of integrity or efficiency . Cutting one in half halves its energy production. Stacking them multiplies power output. Early prototypes of the device are able to produce 2.5 volt s of electricity from a sample the size of a postage stamp.
The devices are formed by combining cellulose with an infusion of aligned carbon nanotubes that are each approximately one millionth of a centimeter thick. The carbon is what gives the batteries their black color. These tiny filaments act like the electrode s found in a traditional battery, conducting electricity when the paper comes into contact with an ionic liquid solution. Ionic liquids contain no water, which means that there is nothing to freeze or evaporate in extreme environmental conditions. As a result, paper batteries can function between -75 and 150 degrees Celsius.
One method of manufacture, developed by scientists at Rensselaer Polytechnic Institute and MIT, begins with growing the nanotubes on a silicon substrate and then impregnating the gaps in the matrix with cellulose. Once the matrix has dried, the material can be peeled off of the substrate, exposing one end of the carbon nanotubes to act as an electrode . When two sheets are combined, with the cellulose sides facing inwards, a supercapacitor is formed that can be activated by the addition of the ionic liquid. This liquid acts as an electrolyte and may include salt-laden solutions like human blood, sweat or urine. The high cellulose content (over 90%) and lack of toxic chemicals in paper batteries makes the device both biocompatible and environmentally friendly, especially when compared to the traditional lithium ion battery used in many present-day electronic devices and laptops.
Widespread commercial deployment of paper batteries will rely on the development of more inexpensive manufacturing techniques for carbon nanotubes. As a result of the potentially transformative applications in electronics, aerospace, hybrid vehicles and medical science, however, numerous companies and organizations are pursuing the development of paper batteries. In addition to the developments announced in 2007 at RPI and MIT, researchers in Singapore announced that they had developed a paper battery powered by ionic solutions in 2005. NEC has also invested in R & D into paper batteries for potential applications in its electronic devices.
While a conventional battery contains a number of separate components, the paper battery integrates all of the battery components in a single structure, making it more energy efficient.
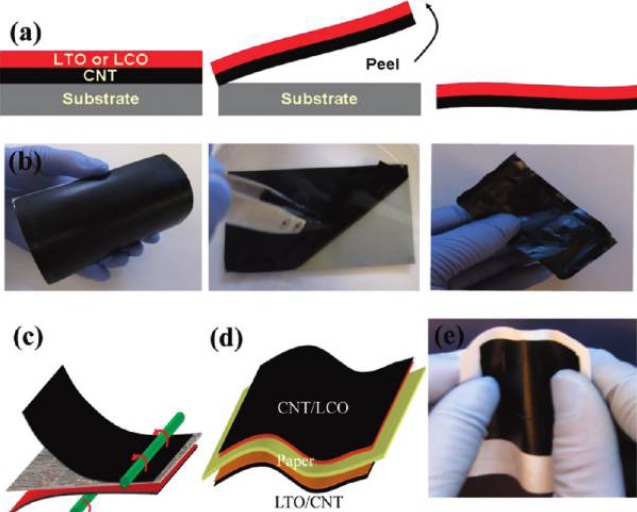
The cross section of the laminated Li-ion paper battery, with the CNT/LTO/paper/LCO/CNT structure, was examined with SEM. Figure 2a reveals the surface morphology of Xerox paper, with large fibers (_20 _m diameter) and surface roughness (peak to valley is _10 _m). Xerox paper lacks microsize holes, which makes it an excellent separator for Li-ion batteries with the laminated electrode films. We tried coating battery electrode materials with the same slurries directly onto either side of Xerox paper, and we found occasional shorting of the device due to the leakage of battery electrode materials through paper.
A paper battery is a battery engineered to use a paper-thin sheet of cellulose infused with aligned carbon nanotubes. nanotubes act as electrodes; allowing the storage devices to conduct electricity
Functions as both a lithium-ion battery and a super capacitor, can provide a long, steady power output comparable to a conventional battery, as well as a supercapacitor’s quick burst of high energy
Integrates all of the battery components in a single structure, making it more energy efficient.
Paper battery extreme flexibility; the sheets can be rolled, twisted, folded, or cut into numerous shapes with no loss of integrity or efficiency, or stacked, like printer paper (or a Voltaic pile), to boost total output.
Can be made in a variety of sizes, from postage stamp to broadsheet.
The paper-like quality of the battery combined with the structure of the nanotubes embedded within gives them their light weight and low cost, making them attractive for portable electronics, aircraft, automobiles, and toys
Ability to use electrolytes in blood make them potentially useful for medical devices such as pacemakers & do not contain any toxic materials and can be biodegradable; a major drawback of chemical cells
While a conventional battery contains a number of separate components, the paper battery integrates all of the battery components in a single structure, making it more energy efficient.
The research appears in the Proceedings of the National Academy of Sciences (PNAS).
"Think of all the disadvantages of an old TV set with tubes," said Professor Linhardt, from the New York-based institute, who co-authored a report into the technology.
"The warm up time, power loss, component malfunction; you don't get those problems with integrated devices. When you transfer power from one component to another you lose energy. But you lose less energy in an integrated device."
The battery contains carbon nanotubes, each about one millionth of a centimetre thick, which act as an electrode. The nanotubes are embedded in a sheet of paper soaked in ionic liquid electrolytes, which conduct the electricity.
The flexible battery can function even if it is rolled up, folded or cut.
Although the power output is currently modest, Professor Linhardt said that increasing the output should be easy.
| Are you interested in this topic.Then mail to us immediately to get the full report.
email :- contactv2@gmail.com |