





Published on Feb 14, 2025
In an era of climate change, alternate energy sources are desired to replace oil and carbon resources. Subsequently, climate change effects in some areas and the increasing production of biofuels are also putting pressure on available water resources. Microbial Fuel Cells have the potential to simultaneously treat wastewater for reuse and to generate electricity; thereby producing two increasingly scarce resources.
While the Microbial Fuel Cell has generated interest in the wastewater treatment field, knowledge is still limited and many fundamental and technical problems remain to be solved Microbial fuel cell technology represents a new form of renewable energy by generating electricity from what would otherwise be considered waste, such as industrial wastes or waste water etc. A microbial fuel cell [Microbial Fuel Cell] is a biological reactor that turns chemical energy present in the bonds of organic compounds into electric energy, through the reactions of microorganism in aerobic conditions.
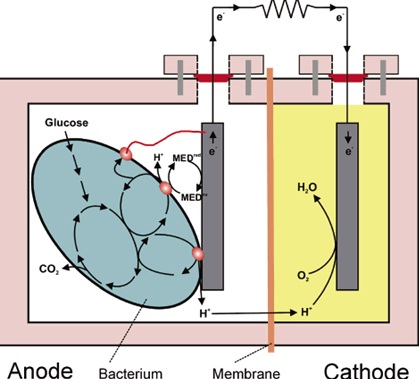
Microbial fuel cell consists of anode and cathode, connected by an external circuit and separated by Proton Exchange Membrane. Anodic material must be conductive, bio compatible, and chemically stable with substrate. Metal anodes consisting of noncorrosive stainless steel mesh can be utilized, but copper is not useful due to the toxicity of even trace copper ions to bacteria. The simplest materials for anode electrodes are graphite plates or rods as they are relatively inexpensive, easy to handle, and have a defined surface area. Much larger surface areas are achieved with graphite felt electrodes
The most versatile electrode material is carbon, available as compact graphite plates, rods, or granules, as fibrous material (felt, cloth, paper, fibers, foam), and as glassy carbon
Proton Exchange Membrane is usually made up of NAFION or ULTREX.
Microbial Fuel Cells utilise microbial communities to degrade organics found within wastewater and theoretically in any organic waste product; converting stored chemical energy to electrical energy in a single step.
Oxygen is most suitable electron acceptor for an microbial fuel cell due to its high oxidation potential, availability, sustainability and lack of chemical waste product, as the only end product is water.
CH 3 COO - + H 2 O 2CO 2 + 2H + +8e -
O 2 + 4e - + 4 H + 2 H 2 O
Electrons produced by bacteria from these substrates are transferred to anode (negative terminal) and flow to the cathode ( positive terminal) linked by a conductive material.
Protons move to cathodic compartment through Proton Exchange Membrane and complete the circuit. Microbial fuel cells use inorganic mediators to tap into the electron transport chain of cells and steal the electrons that are produced. The mediator crosses the outer cell lipid membranes and plasma wall; it then begins to liberate electrons from the electron transport chain that would normally be taken up by oxygen or other intermediates. The now-reduced mediator exits the cell laden with electrons that it shuttles to an electrode where it deposits them; this electrode becomes the electro-generic anode (negatively charged electrode). The release of the electrons means that the mediator returns to its original oxidised state ready to repeat the process. It is important to note that this can only happen under anaerobic conditions, if oxygen is present then it will collect all the electrons as it has a greater electronegativity than the mediator.
To assess bacterial electricity generation, metabolic pathways governing microbial electron and proton flows must be determined. In addition to the influence of the substrate the potential of the anode will also determine the bacterial metabolism. Increasing MFC current will decrease the potential of the anode, forcing the bacteria to deliver the electrons through more-reduced complexes. The potential of the anode will therefore determine the redox potential of the final bacterial electron shuttle, and therefore, the metabolism. Several different metabolism routes can be distinguished based on the anode potential: high redox oxidative metabolism; medium to low redox oxidative metabolism; and fermentation.
Hence, the organisms reported to date in MFCs vary from aerobes and facultative anaerobes towards strict anaerobes. At high anodic potentials, bacteria can use the respiratory chain in an oxidative metabolism. Electrons and, concomitantly, protons can be transported through the NADH dehydrogenase, ubiquinone, coenzyme Q or cytochrome.
The use of this pathway was investigated. They observed that the generation of electrical current from an MFC was inhibited by various inhibitors of the respiratory chain. The electron transport system in their MFC used NADH dehydrogenase, Fe/S (iron/sulphur) proteins and quinines as electron carriers, but does not use site 2 of the electron transport chain or the terminal oxidase. Processes using oxidative phosphorylation have regularly been observed in MFCs, yielding high energy efficiencies of up to 65%.
Examples are consortia containing Pseudomonas aeruginosa, Enterococcus faecium and Rhodoferax ferrireducens. An overview of different bacterial species and their (putative) electron transport pathway is given in. If the anode potential decreases in the presence of alternative electron acceptors such as sulphate, the electrons are likely to be deposited onto these components. Methane production has repeatedly been observed when the inoculum was anaerobic sludge [, indicating that the bacteria do not use the anode. If no sulphate, nitrate or other electron acceptors are present, fermentation will be the main process when the anode potential remains low. For example, during fermentation of glucose, possible reactions can be:
C6H12O6 + 2 H2O 4H2 + 2CO2 + 2C2H4O2
C6H12O6 2 H2 + 2CO2 + C4H8O2
This shows that a maximum of one-third of a hexose substrate electrons can theoretically be used to generate current, whereas two thirds remain in the produced fermentation products such as acetate and butyrate.The one-third of the total electrons are possibly available for electricity generation because the hydrogenases, which generally use the electrons to produce hydrogen gas, are often situated at places on the membrane surface that are accessible from outside by mobile electron shuttles or that connect directly to the electrode. As repeatedly observed, this metabolic type can imply a high acetate or butyrate production. This pathway is further substantiated by the significant hydrogen production observed when MFC enriched cultures are incubated anaerobically in a separate fermentation test.
Some bacterial species in MFCs, of which metal-reducing bacterial are the most important, have recently been reported to directly transfer electrons to the anode. Metal-reducing bacteria are commonly found in sediments, where they use insoluble electron acceptors such as Fe (III) and Mn (IV). Specific cytochromes at the outside of the cell membrane make Shewanella putrefaciens electrochemically active in case it is grown under anaerobic conditions. The same holds true for bacteria of the family Geobacteraceae, which have been reported to form a biofilm on the anode surface in MFCs and to transfer the electrons from acetate with high efficiency.
Rhodoferax species isolated from an anoxic sediment were able to efficiently transfer electrons to a graphite anode using glucose as a sole carbon source. Remarkably, this bacterium is the first reported strain that can completely mineralize glucose to CO2 while concomitantly generating electricity at 90% efficiency. In terms of performance, current densities in the order of 0.2-0.6mA and a total power density of 1-17 mW/m2 graphite surface have been reported for Shewanella putrefaciens, Geobacter sulfurreducens and Rhodoferax ferrireducens at conventional (woven) graphite electrodes (Bond and Lovley 2003, Chaudhuri and Lovley 2003, Kim et al. 2002) (Table 20.4). However, in case woven graphite in the Rhodoferax study was replaced by highly porous graphite electrodes, the current and power output was increased up to 74 mA/m2 and 33 mW/m2, respectively.
Although these bacteria generally show high electron transfer efficiency, they have a slow growth rate, a high substrate specificity (mostly acetate or lactate) and relatively low energy transfer efficiency compared to mixed cultures. Furthermore, the use of a pure culture implies a continuous risk of contamination of the MFCs with undesired bacteria.
wikipedia.org
| Are you interested in this topic.Then mail to us immediately to get the full report.
email :- contactv2@gmail.com |