





Published on Feb 14, 2025
Scientific research in the area of semiconducting organic materials as the active substance in light emitting diodes (LEDs) has increased immensely during the last four decades. Organic semiconductors was first reported in the 60:s and then the materials where only considered to be merely a scientific curiosity. (They are named organic because they consist primarily of carbon, hydrogen and oxygen.).
However when it was recognized in the eighties that many of them are photoconductive under visible light, industrial interests were attracted. Many major electronic companies, such as Philips and Pioneer, are today investing a considerable amount of money in the science of organic electronic and optoelectronic devices. The major reason for the big attention to these devices is that they possibly could be much more efficient than todays components when it comes to power consumption and produced light.
Common light emitters today, Light Emitting Diodes (LEDs) and ordinary light bulbs consume more power than organic diodes do. And the strive to decrease power consumption is always something of matter. Other reasons for the industrial attention are i.e. that eventually organic full color displays will replace todays liquid crystal displays (LCDs) used in laptop computers and may even one day replace our ordinary CRT-screens.
Organic light-emitting devices (OLEDs) operate on the principle of converting electrical energy into light, a phenomenon known as electroluminescence. They exploit the properties of certain organic materials which emit light when an electric current passes through them. In its simplest form, an OLED consists of a layer of this luminescent material sandwiched between two electrodes. When an electric current is passed between the electrodes, through the organic layer, light is emitted with a color that depends on the particular material used. In order to observe the light emitted by an OLED, at least one of the electrodes must be transparent.
When OLEDs are used as pixels in flat panel displays they have some advantages over backlit active-matrix LCD displays - greater viewing angle, lighter weight, and quicker response. Since only the part of the display that is actually lit up consumes power, the most efficient OLEDs available today use less power.
Based on these advantages, OLEDs have been proposed for a wide range of display applications including magnified microdisplays, wearable, head-mounted computers, digital cameras, personal digital assistants, smart pagers, virtual reality games, and mobile phones as well as medical, automotive, and other industrial applications.
Electronically, OLED is similar to old-fashioned LEDs -- put a low voltage across them and they glow. But that's as far as the similarity goes: instead of being made out of semiconducting metals, OLEDs are made from polymers, plastics or other carbon-containing compounds. These can be made very cheaply and turned into devices without all the expensive palaver that goes with semiconductor fabrication.
Light-emitting diodes, based upon semiconductors such as Gallium Arsenide, Gallium Phosphide, and, most recently, Gallium Nitride, have been around since the late '50s. They are mostly used as indicator lamps, although they were used in calculators before liquid crystals, and are used in large advertising signs, where they are valued for very long life and high brightness. Such crystalline LEDs are not inexpensive, and it is very difficult to integrate them into small high-resolution displays.
The operation of an LED is based upon the fact that semiconductors can be of two types, p-type or n-type, depending upon whether dopants pull electrons out of the crystal, forming "holes", or add electrons. An LED is formed when p-type and n-type materials are joined. When a voltage is applied, causing electrons to flow through the structure, electrons flow into the p-type material, and holes flow into the n-type material. An electron-hole combination is unstable; there is too much potential energy to be released. As a result, they combine and release the energy in the form of light. This can be a very efficient way to convert electricity to light.
There is a wide class of organic compounds, called conjugated organics or conjugated polymers, which have many of the characteristics of semiconductors. They have energy gaps of about the same magnitude, they are poor conductors without dopants, and they can be doped to conduct by electrons (n-type) or holes (p-type). Initially, these materials were used as photoconductors, to replace inorganic semiconductor photoconductors, such as selenium, in copiers. About fifteen years ago it was discovered that, just as with crystalline semiconductors, p-type and n-type organic materials can be combined to make light-emitting diodes whereby a current passing through a simple layered structure produces visible light with high efficiency.
Since light-emitting diodes, as their name suggests, actually generate their own light while using very little battery power, they have long been viewed as an obviously better way to create displays. Unfortunately, while conventional L.E.D.'s work well in giant screens and advertising displays like those in Times Square, they cannot easily be used to create small, high-resolution screens for portable computers.
OLED is an emissive display technology based on thin organic light-emitting films. Like conventional inorganic light emitting diodes (LED), OLED requires a low-drive voltage to produce bright visible light. But unlike discrete LEDs, which have crystalline origins, thin film-based OLEDs have area emitters that can easily be patterned to produce flat-panel displays. Because OLEDs are self-luminous, backlights are not required as in liquid-crystal displays (LCDs). OLEDs have very low power requirements and are thin, bright and efficient.
Because crystalline order is not required, organic materials, both molecular and polymeric, can be deposited far more cheaply than the inorganic semiconductors of conventional LED’s. Patterning is also easier, and may even be accomplished by techniques borrowed from the printing industry. Displays can be prepared on flexible, transparent substrates such as plastic. These characteristics form the basis for a display technology that can eventually replace even paper, providing the same resolution and reading comfort in a long-lived, fully reusable (and eventually recyclable) digital medium.
Also OLEDs are much more efficient than todays components when it comes to power consumption and produced light. Common light emitters today, Light Emitting Diodes (LEDs) and ordinary light bulbs consume more power, To tell something about the efficiency of components we will use the concept of Quantum Efficiency (QE), which is defined as the relation between photons produced and electrons injected. To achieve a high QE for a light bulb it would be necessary to change the relation between produced heat and produced light. To increase the QE for a LED it is necessary to limit the absorption of the photons produced. It has been generally observed that organic devices can and will produce high quantum efficiencies than organic diodes do.
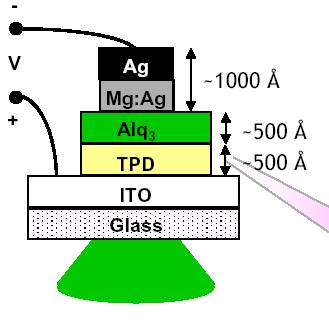
The organic light emitting diode (OLED) is a p-n diode, in which charge-carriers (e-h pairs) recombine to emit photons in an organic layer. The thickness of this layer is approximately 100 nm (experiments have shown that 70 nm is an optimal thickness). When an electron and a hole recombines, an excited state called an exciton is formed. Depending on the spin of the e-h pair, the exciton is either a singlet or a triplet. An electron can have two different spins, spin up and spin down. When the spin of two particles is the same, they are said to be in a spin-paired, or a triplet state, and when the spin is opposite they are in a spin-paired singlet state.
On the average, one singlet and three triplets are formed for every four electron- hole pairs, and this is a big inefficiency in the operation of the diodes. A singlet state decays very quickly, within a few nanoseconds, and thereby emits a photon in a process called fluorescence. A triplet state, however, is much more long-lived (1 ms - 1 s), and generally just produce heat. One method of improving the performance is to add a phosphorescent material to one of the layers in the OLED. This is done by adding a heavy metal such as iridium or platinum. The exciton can then transfer it's energy to a phosphorescent molecule which in turn emits a photon. It is however a problem that few phosphorescent materials are efficient emitters at room temperature.
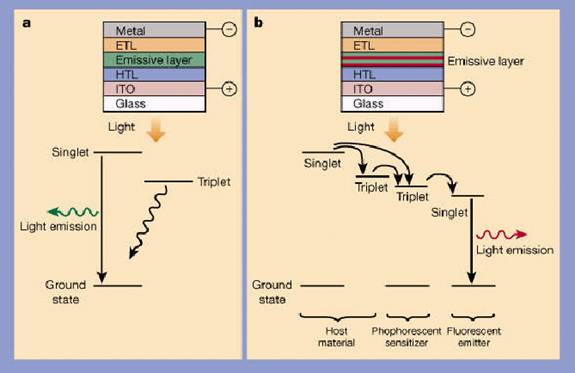
There have been devices manufactured which transforms both singlet and triplet states in a host to a singlet state in the fluorescent dye. This is done by using a phosphorescent compound which both the singlets and triplets transfer their energy to, after which the compound transfer its energy to a fluorescent material which then emits light.
Using one organic layer has some problems associated with it. The electrodes energy levels have to be matched very closely, otherwise the electron and hole currents will not be properly balanced. This leads to a waste in energy since charges can then pass the entire structure without recombining, and this lowers the efficiency of the device. With two organic layers, the situation improves dramatically. Now the different layers can be optimized for the electrons and holes respectively. The charges are blocked at the interface of the materials, and “waits” there for a “partner”.
Considerably better balance can be achieved by using two organic layers one of which is matched to the anode and transports holes with the other optimized for electron injection and transport. Each sign of charge is blocked at the interface between the two organic layers and tend to "wait" there until a partner is found. Recombination therefore occurs with the exciton forming in the organic material with the lower energy gap. The fact that it forms near the interface is also beneficial in preventing quenching of the luminescence that can occur when the exciton is near one of the electrodes.
Another improvement is to introduce a third material specifically chosen for its luminescent efficiency. Now the three organic materials can be separately optimized for electron transport, for hole transport and for luminescence.
| Are you interested in this topic.Then mail to us immediately to get the full report.
email :- contactv2@gmail.com |