





Published on Apr 02, 2024
Smart materials respond to things that happen around them. This activity demonstrates the properties of one smart material: Nitinol. Nitinol is a mixture of two different metals: nickel and titanium. Nitinol stands for Ni (nickel), Ti (titanium), and Naval Ordnance Laboratory, the place where it was discovered in 1965. What makes Nitinol so smart? Find out! In this activity, you will demonstrate the shape memory properties of Nitinol by shaping and heating samples.
In this activity, you will demonstrate the shape memory properties of Nitinol by shaping and heating samples.
• Piece of Nitinol wire1
• Hairdryer or hot water
• Bowl
• Paper clip or other wire (optional)
Use your senses to observe the Nitinol wire. Is it soft? Hard? Stretchy? What materials is it like? How would it be different if it were thinner? Thicker?
• Bend the wire into a shape.
• Try to straighten the wire back out. Give up?

• Put the wire in the bowl, and blow the hairdryer on it. What happens? If nothing happens, move the hairdryer closer to the wire. Nitinol can get very hot, so please don’t hold the wire while heating it.
If you bend a paper clip, or other wire out of shape, and then heat it up, what happens? Try it! How does Nitinol behave differently from other wires? What are the differences between Nitinol and other wires?
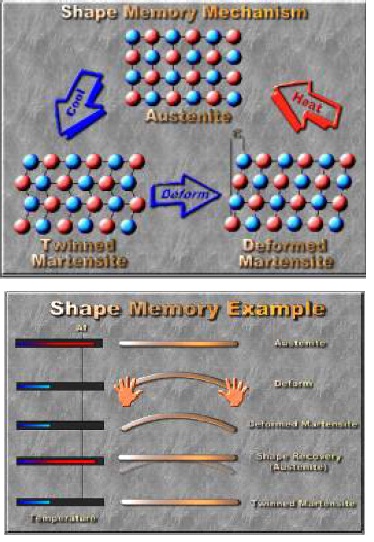
Everything around us is made of tiny building blocks called atoms. Nitinol is made up of two kinds of atoms: Nickel atoms and Titanium atoms. They are arranged in an organized pattern called a crystal structure. Most solids have a crystal structure, but Nitinol is special because it has two different crystal structures, also called solid phases. At colder temperatures, Nitinol’s atoms are in one arrangement, called martensite. At higher temperatures, Nitinol’s atoms are in a slightly different crystal structure, called austenite. When you heat Nitinol, you give the atoms energy to move from the martensite structure to the austenite structure, and as it cools back down, they move back.
The atoms in the Nitinol move just a little bit, but this makes a huge difference in how the metal feels and behaves. At lower temperatures, Nitinol is soft and easy to bend. At hotter temperatures, it is stiff and springy. How does that make Nitinol a smart material? When it’s in its low-temperature structure, when you heat it up, it will change to its high-temperature structure. It bounces back to its original shape when that change happens. When you heated the wire up using the hair dryer, you caused the Nitinol to change from one phase to the other, and it went back to the shape it was in before you bent it. This gives Nitinol its other name, memory metal.
You can use electricity to heat up the wire, just like electricity heats up the coils in your toaster. Electricity is used to change Nitinol’s shape when it is used as the legs of robots, or in toys, statues or machinery.
• Piece of Nitinol wire
• 6 Volt lantern battery
• 2 leads with alligator clips on both ends
Parents, please supervise children.
• Bend the wire.
• Clip one end of an alligator clip lead onto the positive terminal of the battery. Be careful not to pinch fingers in clips.
• Attach the other end of the lead to one end of your bent piece of Nitinol.
• Attach one end of the other lead to the other end of the Nitinol.
• Nitinol can get very hot when using electricity. Don’t touch the wire as it is heating. Hold the alligator clips by the insulated covers. Gently touch the other end of that alligator clip to the negative battery terminal. What happens to the wire? Compare it to when you blow a hairdryer on a piece of Nitinol.
What other materials in your house have memory? A rubber band will go back to its original size when you stretch it and then let go. What happens if you stretch a rubber band around a large object and leave it for awhile? Does it go back to its original size? Can you think of any other materials in your house that have memory?
Nitinol reacts to a change in its environment – temperature - so it is a smart material. Nitinol and some other metal mixtures have shape memory, which has made them useful in a lot of inventions, like wires for braces, staples that hold broken bones together, coffee pots that turn off when the water is hot enough, and trick spoons that bend when you try to stir your tea!
1. Teaching General Chemistry: A Materials Science Companion. Arthur B. Ellis, Margret J. Gesselbracht, Brian J. Johnson, George C. Lisensky and William R. Robinson. Published by the American Chemical Society, 1993.
2. Nitinol Information, from the Images SI, Inc. website: http://www.imagesco.com/articles/nitinol/01.html
3. Nitinol Technical Data, from Johnson Matthey’s website: http://www.jmmedical.com/html/ nitinol_technical_information.html