





Published on Apr 02, 2024
Measuring the Amount of Acetic Acid In Vinegar by Titration with an Indicator Solution. The goal of this project is to determine the amount of Acetic Acid in different types of vinegar using titration with a coloured pH indicator to determine the endpoint.
Vinegar is a solution made from the fermentation of ethanol (CH3CH2OH), which in turn was previously fermented from sugar. The fermentation of ethanol results in the production of acetic acid (CH3COOH). There are many different types of vinegar, each starting from a different original sugar source (e.g., rice, wine, malt, etc.). The amount of acetic acid in vinegar can vary, typically between 4 to 6% for table vinegar, but up to three times higher (18%) for pickling vinegar. In this project, we will determine the amount of acid in different vinegars using titration, a common technique in chemistry. Titration is a way to measure the unknown amount of a chemical in a solution (the titrant) by adding a measured amount of a chemical with a known concentration (the titrating solution). The titrating solution reacts with the titrant, and the endpoint of the reaction is monitored in some way.
The concentration of the titrant can now be calculated from the amount of titrating solution added, and the ratio of two chemicals in the chemical equation for the reaction. To measure the acidity of a vinegar solution, we can add enough hydroxyl ions to balance out the added hydrogen ions from the acid.
The hydroxyl ions will react with the hydrogen ions to produce water. In order for a titration to work, we need three things:
1. a titration solution (contains hydroxyl ions with a precisely known concentration),
2. a method for delivering a precisely measured volume of the titrating solution, and
3. a means of indicating when the endpoint has been reached.
For the titrating solution, we’ll use a dilute solution of Sodium Hydroxide (NaOH). Sodium Hydroxide is a strong base, which means that it dissociates completely in water. So for every NaOH molecule that we add to the solution, we can expect to produce a hydroxyl ion. To dispense an accurately measured volume of the titrating solution, we will use a burette. A burette is a long tube with a valve at the bottom and graduated markings on the outside to measure the volume contained in the burette.
The burette is mounted on a ring stand, directly above the titrant solution (as shown in the picture). Solutions in the burette tend to creep up the sides of the glass at the surface of the liquid. This is due to the surface tension of water. The surface of the liquid thus forms a curve, called a meniscus. To measure the volume of the liquid in the burette, always read from the bottom of the meniscus. In this experiment, we will use an indicator solution called phenolphthalein. Phenolphthalein is colourless when the solution is acidic or neutral.
When the solution becomes slightly basic, Phenolphthalein turns pinkish, and then light purple as the solution becomes more basic. So when the vinegar solution starts to turn pink, we know that the titration is complete.
To do this experiment we will need the following materials and equipment:
Vinegar, three different types.
Distilled water
Small funnel
0.5% Phenolphthalein solution in alcohol (pH indicator solution)
0.1 M sodium hydroxide solution
125 mL Conical flask
25 or 50 mL burette
10 mL graduated cylinder
Ring stand
Burette clamp
Required amount of sodium hydroxide (NaOH) can be calculated using the following formula:
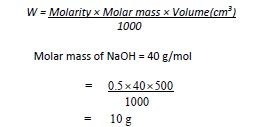
The acetic acid content of a vinegar may be determined by titrating a vinegar sample with a solution of sodium hydroxide of known molar concentration (molarity).
CH3COOH + NaOH --> CH3COONa + H2O
(Acid) + (Base) --> (Salt) + (Water)
At the end point in the titration stoichiometry between the both solution lies in a 1:1 ratio.
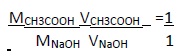
Strength of acid in vinegar can be determined by the following formula:
Strength of acetic acid = MCH3COOH × 60
Performing the Titration 1. Pour 1.5 ml of vinegar in an Conical flask.
2. Add distilled water to dissolve the vinegar so that the volume of the solution becomes 20 mL.
3. Add 3 drops of 0.5% phenolphthalein solution.
4. Use the burette clamp to attach the burette to the ring stand. The opening at the bottom of the burette should be just above the height of the conical flask we used for the solution of vinegar and phenolphthalein solution.
5. Use a funnel to fill the burette with a 0.1 M solution of sodium hydroxide.
6. Note the starting level of the sodium hydroxide solution in the burette. Put the vinegar solution to be titrated under the burette.
7. Slowly drip the solution of sodium hydroxide into the vinegar solution. Swirl the flask gently to mix the solution, while keeping the opening underneath the burette.
8. At some point we will see a pink colour in the vinegar solution when the sodium hydroxide is added, but the colour will quickly disappear as the solution is mixed. When this happens, slow the burette to drop-by-drop addition.
9. When the vinegar solution turns pink and remains that colour even with mixing, the titration is complete. Close the tap (or pinch valve) of the burette.
10. Note the remaining level of the sodium hydroxide solution in the burette. Remember to read from the bottom of the meniscus.
11. Subtract the initial level from the remaining level to figure out how much titrating solution we have used.
12. For each vinegar that we test, repeat the titration at least three times.
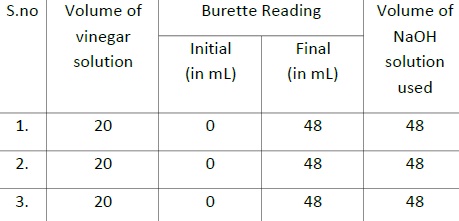
Strength of acetic acid in household vinegar = 40.5 g/L.
Strength of acetic acid in wine vinegar = 72 g/L.
Strength of acetic acid in fruit vinegar = 48 g/L.
Transference of measured vinegar into a measuring flask should be done very carefully.
Measuring must be performed carefully.
Look at the meniscus of solution at eye level to avoid parallax.
Look at the lower meniscus in the light coloured solution and upper meniscus in the dark coloured solution because of visibility.
Do not forget to add distilled water to the vinegar.
NCERT Chemistry- XII
Comprehensive Practical Chemistry- XII
www..scribd..com