





Published on Nov 30, 2023
The objective of this work was to explore the feasibility of generating a fiber-latex- TiO2-composite material that could be used as an additive in the papermachine wet end. By initially binding a cationic latex and subsequently anionic TiO2 to the fiber surface and then curing the latex, it was envisioned that the pigment would be strongly retained in a latex film on the fiber surface. This novel additive could be utilized in papermaking operations achieving high TiO2 retention and substantial cost-savings. Dispersion turbidity and microscopy were used to monitor experiments.
Data were generated on the interaction between fiber and cationic latex, and a fiber-latex intermediate and TiO2. Retention levels of TiO2 in the range of the target value were achieved, and stability tests proved the material resistant to normal pH and shear stresses. However, the cured composite proved difficult to redisperse and a different approach for curing must be explored.
As light travels from one medium to another, its path is bent according to the difference in refractive index of the two media and the light is scattered. The incident light undergoes reflection by the surface and penetration into the sheet. The penetrating light is either absorbed or scattered by the particles in the structure. Light scattering in a non-filled uncoated paper will consequently depend on fiber and sheet properties, such as pulping method, sheet bulk, homogeneity and grammage. TiO2, improves sheet opacity when contained in paper.
Sheet opacity is defined as a “contrast ratio” 100(R0/R¥), where R0 is the reflectance of a single sheet backed by a non-reflecting surface and R¥ is the reflectance of an infinite number of sheets of the same material. TAPPI similarly defines opacity as a “contrast ratio” 100*(R0/R0.89), where R0 is defined as above and R0.89 is the reflectance of a sheet backed by a white material with 89 % reflectance (3,7). Thus, higher reflectance by the sheet surface and scattering of light within the sheet will result in higher opacities. Based on these observations, sheet opacites can be enhanced by producing layered sheets, incorporating filler particles into the fiber web or applying a pigment coating to the sheet. Layered sheets, mainly used in board applications, can include a dark central layer yielding high opacity (5).
Pigment fillers generate a larger surface area within the sheet and introduce interfaces with larger differences in refractive index relative to the inherent ones between woodparticles and air. Pigment coatings drastically alter the sheet surface, essentially creating a more dense layered structure, improving among other things sheet opacity. However, fillers and coatings have negative effects on other paper properties like strength and bulk. Improper application or poor control can also have severe effects on the manufacturing process and final product quality.
In terms of electronic structure (3d24s2), titanium is the simplest of the transition metals. It constitutes 0.6 % of the earth’s crust, mainly encountered as ores of FeTiO3 (ilmenite) and the binary metal oxides, TiO2 (rutile, anatase and brookite). The rutile polymorph is the most common of the TiO2 species, and accounts for about 90% of the TiO2 sold world wide (8,9). Global production in 1999 was 3,600,000 metric tons. About 396,000 metric tons (11%) went into the paper market. Industrial application in paints and plastics accounted for the bulk of the remaining consumption (9). Rutile is used in all industrial applications, and exclusively in paints and plastic due to its higher refractive index and non-reactive nature. Anatase is found in specialty products like food, cosmetics, and fibers. Both are used in paper with rutile having a more dominant role due to its better optical properties. The properties of synthetic titanium oxides are modified through the manufacturing process and are different from the inherent properties of the naturally occurring minerals.
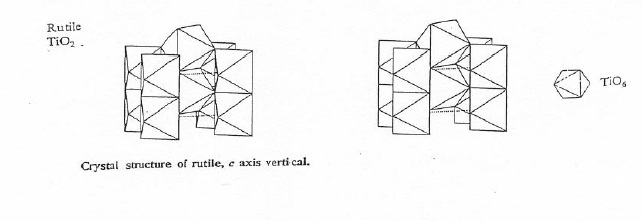
Rutile and anatase have very similar physical and chemical properties, but represent two different crystal structures due to differences in atomic arrangement. Both structures exhibit octahedral coordination of titanium by oxygen with tetragonal symmetry. However, as shown in Figure 2.4, rutile has rows of edge-sharing octahedra in the c direction which are connected by corner sharing in the a and b directions. Meanwhile, as shown in Figure 2.5, anatase displays a more complicated connectivity. The structure has a three dimensional topology of edge-sharing octahedra in all directions, forming a zigzag pattern through the crystal plane
TiO2 belongs to a group of compounds generally referred to as d0 oxides, indicating a normally empty d-orbital in the metal atom. The titanium(+IV) valence orbital has a filled shell electron configuration. Since there are no electrons available for transfer during chemisorption the surface is unreactive. However, when molecular surface defects due to oxygen-vacancy are present, d-orbitals of adjacent cations can be partially occupied. Points of oxygen deficiency, i.e. a reduced surface, will therefore increase the reactivity of the surface (10,12). A good survey of adsorption on TiO2 surfaces is given by Heinrich and Cox (11). One characteristic of TiO2 with implications for its general utilization in paper machine wet-end applications is that its particle surface charge is variable and pH-dependent in aqueous dispersions. The TiO2 point of zero charge, PZC, the pH at which the pigment surface charge equals zero, is pH 6.25.
Alkaline papermaking has a wet-end aqueous environment with pHs substantially greater than 6.25, TiO2 particles carry a negative surface charge, and will, therefore, from this point forward be treated as an anionic additive (9). Wet-end additive retention largely depends on electrostatic interactions between fiber, fiber derivatives, and additives like filler particles. These electrostatic interactions are highly dependent on the surface charge of the involved species. Excluding quaternary amines, the degree of surface charge varies with pH for most components. Controlling the addition points and pH in the stock flow is therefore imperative for optimum additive retention
Scott addresses some of the most important factors affecting light scattering in paper (4). Pertaining to fillers, the particle size, refractive index and specific surface area are key features determining a filler’s effectiveness as an opacifier and brightener. The most efficient light-scattering is found in spherical particles with diameters that are about 0.25 mm, or about half the wave-length of light. Furthermore, the greater the number of optical interfaces within a paper, the greater the light-scattering and resulting opacity. As seen in Table 2.2, the refractive index of TiO2 is the highest encountered among pigments used in the paper industry. The particle size-distribution in commercial TiO2 is also narrow and close to the optimal 0.25 mm.
Although the specific surface area of the TiO2 particles is not as high as in other fillers, its much higher refractive index renders TiO2 as the best pigment for achieving opacity. In addition to being the best opacifier, TiO2 also has a very high ISO brightness at 96-97 %. Sheet brightness generally increases with increasing filler levels, as long as filler particles do not aggregate extensively. Aggregation results in a decrease in available interfaces where light-scattering can occur. This presents one of the basic problems for the paper maker – achieving the highest possible opacity by increasing filler addition and keeping the particles from aggregating in the sheet. The higher refractive index and brightness of TiO2 allow the paper maker to achieve a higher sheet opacity and brightness at lower loading levels
In addition to optical properties, fillers affect several other sheet properties. For example, fillers affect density, air permeability and smoothness. The level of impact will vary among fillers due to their individual characteristics. For a sheet of specific grammage, tensile and burst decrease with increasing filler levels. The negative impact on sheet strength is greater in the case of TiO2 compared to other fillers due to its smaller particle size and spherical shape. Sizing decreases with increasing filler content, as the internal surface area of the sheet is increased. This can be overcome by increasing the amount of added sizing agent. As shown by the above examples, it is very important to understand how TiO2 interacts with other components in the stock flow and how these interactions affect the final sheet quality
| Are you interested in this topic.Then mail to us immediately to get the full report.
email :- contactv2@gmail.com |